Anyplex™Ⅱ RV16 Detection simultaneously detects and differentiates 16 of the most prevalent respiratory viruses that currently known in the clinical field. Based on Seegene’s proprietary DPO™ and TOCE™ technologies, this multiplex assay performs on real-time PCR instruments and provides infected information of 16 respiratory viruses in a single test with both high sensitivity and specificity.
Key Features and Benefits
-

Multiplex real-time PCR
Detection and differentiation of 16 respiratory viruses in a single reaction
-
Proper patient care
Quick and proper treatment provided by accurate test results
-

Automatic data analyzer
Automated data interpretation and LIS interlocking with Seegene Viewer
-
Whole process validation
Whole process validation from extraction to PCR by whole process control
-

Proper patient care
Quick and proper treatment provided by accurate test results
-
User-friendly workflow
Convenient workflow using Seegene’s automated one platform
-

Automatic data analyzer
Automated data interpretation and LIS interlocking with Seegene Viewer
-

Quantitative analysis
Quantitative analysis by cyclic-CMTA
-
UDG system
Utilization of the UDG system to prevent carry-over contamination
-
Analytes
Panel A
- Adenovirus (AdV)
- Influenza A virus (Flu A)
- Influenza B virus (Flu B)
- Parainfluenza virus 1 (PIV 1)
- Parainfluenza virus 2 (PIV 2)
- Parainfluenza virus 3 (PIV 3)
- Parainfluenza virus 4 (PIV 4)
- Human rhinovirus (HRV)
- Internal Control (IC)
-
Panel B
- Bocavirus 1/2/3/4 (HBoV)
- Coronavirus 229E (229E)
- Coronavirus NL63 (NL63)
- Coronavirus OC43 (OC43)
- Enterovirus (HEV)
- Metapneumovirus (MPV)
- Respiratory syncytial virus A (RSV A)
- Respiratory syncytial virus B (RSV B)
- Internal Control (IC)
-
Specimens
- Nasopharyngeal swab
- Nasopharyngeal aspirate
- Bronchoalveolar lavage
-
Ordering Information
Result
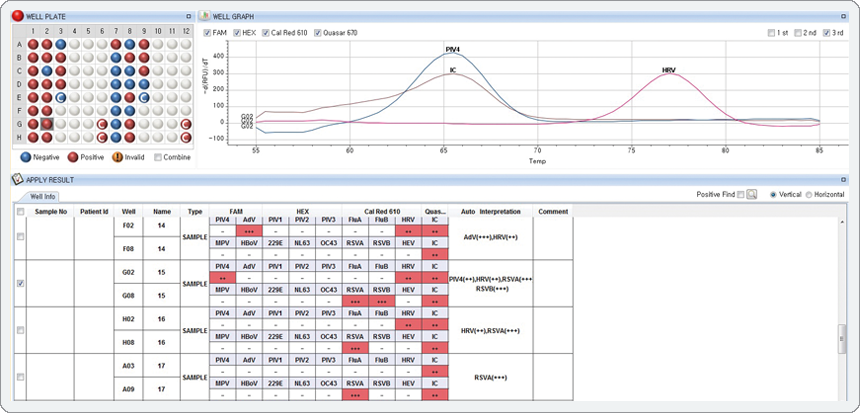
The result represents co-infection that PIV4 is intermediate (++) in the FAM channel, RSV-A is high(+++) and RSA-B is high (+++) in the Cal Red 610 channel.
Publication
Note
[1] For use with Seegene NIMBUS & STARlet only
