Anyplex™II MTB/XDR Detection simultaneously detects and identifies Mycobacterium tuberculosis (MTB) and 13 mutations associated with XDR-TB. Based on Seegene’s proprietary DPO™ and TOCE™ technologies, this assay performs on multiplex real-time PCR instruments and provide prompt diagnosis and appropriate treatment guideline for tuberculosis control.
Key Features and Benefits
-

Multiplex real-time PCR
Simultaneous detection of Mycobacterium tuberculosis and 13 mutations associated with XDR-TB
-
DNA extraction solution
Reagent for DNA Detection is provided, purchase of DNA extraction kit is unnecessary
-
Wide range of specimens
Wide range of applicable specimens
-

Automatic data analyzer
Automated data interpretation and LIS interlocking with Seegene Viewer
-
Fast treatment decision
Fast treatment decision by simultaneous screening and subtyping
-
Prediction of antibiotic resistance
Detection and identification of mutations associated antibiotic resistance for appropriate infection control
-
Whole process validation
Whole process validation from extraction to PCR by whole process control
-

Automatic data analyzer
Automated data interpretation and LIS interlocking with Seegene Viewer
-
Analytes
-
Specimens
- Sputum
- Bronchial washing
- Cultured cell
- Fresh tissue
-
Ordering Information
ProductCat No. / SizeAnyplex™ II MTB/XDR DetectionTB7302Y / 50 rxns
Result
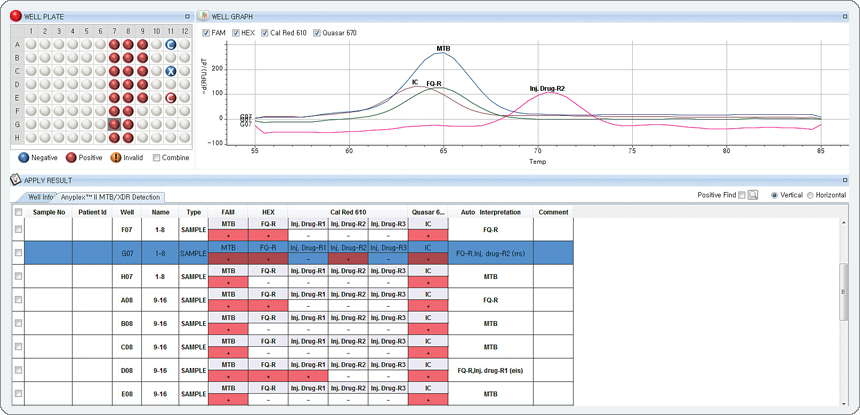
These results indicate the detection of MTB infection in the FAM channel, and the Fluoroquinolone-resistance mutation in the HEX channel and the Injectable drug-resistance-R2* mutation in the Cal Red 610 channel.
(* High level Kanamycin, Amikacin, Capreomycin resistance)
Note
[1] The WTC is designed to be exhibited the same result pattern with drug-susceptible M. tuberculosis sample. The WTC reaction should be always performed in each testing run, and the drug-resistant result of unknown samples is analyzed on the basis of the result of WTC.
[2] Extensively Drug Resistance

