Allplex™ Respiratory Panel 3 is a multiplex one-step real-time RT-PCR assay that detects and identifies 5 causative viral pathogens in respiratory tract infection including Coronavirus. Based on Seegene’s proprietary MuDT™ technology, this assay reports multiple Ct values of each pathogen in a single channel using real-time PCR instrument.
Key Features and Benefits
-

Multiplex real-time PCR
Detection and differentiation of 5 viruses causing respiratory tract infections in a single reaction
-

Proper patient care
Quick and proper treatment provided by accurate test results
-
Powerful performance with unique technology
Multiplex real-time PCR with high sensitivity and specificity by utilization of DPO™ and TOCE™ technologies
-

Multi-Ct in a single channel
Individual Ct value of multiple analytes in a single channel of real-time PCR instrument (MuDT™ Technology)
-

UDG system
Utilization of the UDG system to prevent carry-over contamination
-
Informative assay
Assistant of appropriate treatment and management for co-infection
-

Whole process validation
Whole process validation from extraction to PCR by whole process control
-
User-friendly workflow
Convenient workflow using Seegene’s automated one platform
-

Automatic data analyzer
Automated data interpretation and LIS interlocking with Seegene Viewer
-
Analytes
Allplex™
Respiratory Panel 3
- Bocavirus 1/2/3/4 (HBoV)
- Coronavirus 229E (229E)
- Coronavirus NL63 (NL63)
- Coronavirus OC43 (OC43)
- Human rhinovirus (HRV)
- Internal Control (IC)
-
Specimens
- Nasopharyngeal swab
- Nasopharyngeal aspirate
- Bronchoalveolar lavage
-
Ordering Information
Result
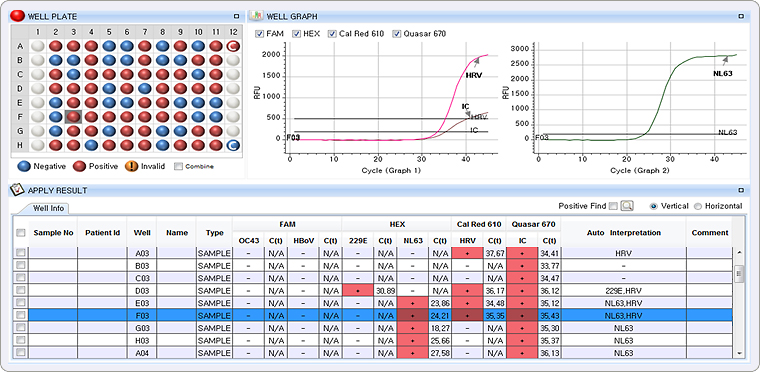
Multiple Ct value in a single channel
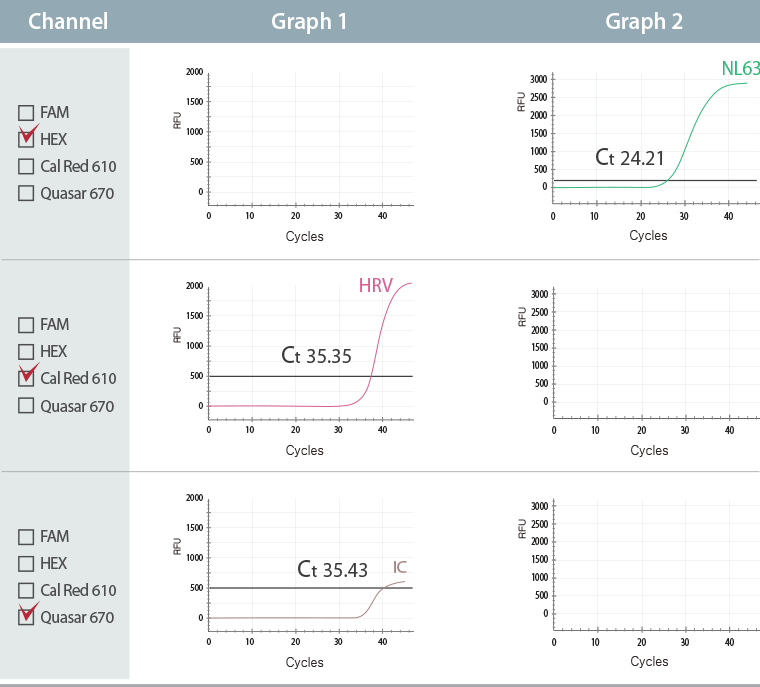
This result represents co-infection of Rhinovirus (HRV) and Coronavirus NL63 (CoV NL63) in Cal Red 610 channel and HEX channel with each Ct value of 35.35 (Graph 1) and 24.21 (Graph 2)
Publication
Note
[1] For use with Seegene NIMBUS & STARlet only
