Allplex™ Gastrointestinal Panel Assays is a multiplex one-step real-time RT-PCR assay that detects and identifies 25 gastrointestinal pathogens including 6 viruses, 13 bacteria and 6 parasites simultaneously. Based on Seegene’s proprietary MuDT™ technology, this assay reports multiple Ct values of each pathogen in a single channel using real-time PCR instrument. Allplex™ Gastrointestinal Panel Assays allows faster, more reliable and comprehensive test results than any other products by combination with Seegene’s automation platforms.
Key Features and Benefits
-

Multiplex real-time PCR
Broad pathogens coverage of 6 viruses, 13 bacteria and 6 parasites causing gastrointestinal pathogens in a single reaction
-
Full coverage of causative pathogens
Comprehensive assay for the detection and identification of 25 (gastrointestinal) pathogens
-

Automatic data analyzer
Automated data interpretation and LIS interlocking with Seegene Viewer
-
Proper patient care
Quick and proper treatment provided by accurate test results
-
Informative assay
Assistant of appropriate treatment and management for co-infection
-

UDG system
Utilization of the UDG system to prevent carry-over contamination
-

Whole process validation
Whole process validation from extraction to PCR by whole process control
-
User-friendly workflow
Convenient workflow using Seegene’s automated one platform
-

Efficient syndromic test
The syndromic screening test for efficient and cost-effectiveness patient care
-

Proper patient care
Accurate test results allow to quick and proper treatment
-

Multi-Ct in a single channel
Individual Ct value of multiple analytes in a single channel of real-time PCR instrument (MuDT™ Technology)
-
Analytes
-
Allplex™
GI-Bacteria(I) Assay- Aeromonas spp. (Aer)
- Campylobacter spp. (Cam)
- Clostridium difficile toxin B (CdB)
- Salmonella spp. (Sal)
- Shigella spp./EIEC[5] (Sh/EI)
- Vibrio spp. (Vib)
- Yersinia enterocolitica (Yer)
- Internal Control (IC)
-
Allplex™
GI-Parasite Assay- Blastocystis hominis (BH)
- Cryptosporidium spp. (CR)
- Cyclospora cayetanensis (CC)
- Dientamoeba fragilis (DF)
- Entamoeba histolytica (EH)
- Giardia lamblia (GL)
- Internal Control (IC)
-
Allplex™
GI-Virus Assay- Adenovirus (AdV)
- Astrovirus (AstV)
- Norovirus GI (NoV-GI)
- Norovirus GII (NoV-GII)
- Rotavirus (RotV)
- Sapovirus (SV)
- Internal Control (IC)
-
Specimen
- Human stool
-
Ordering Information
ProductCat No. / SizeAllplex™ GI-Bacteria(II) AssayAllplex™ GI-Bacteria(I) AssayAllplex™ GI-Parasite AssayAllplex™ GI-Virus Assay
Result
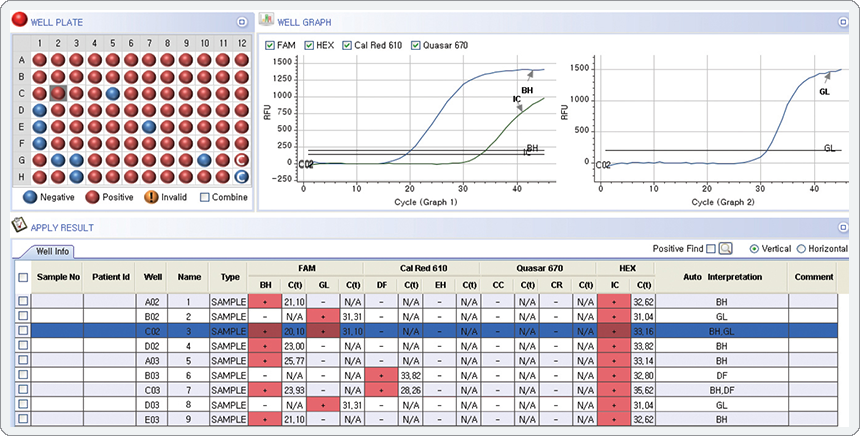
Multiple Ct value in a single channel
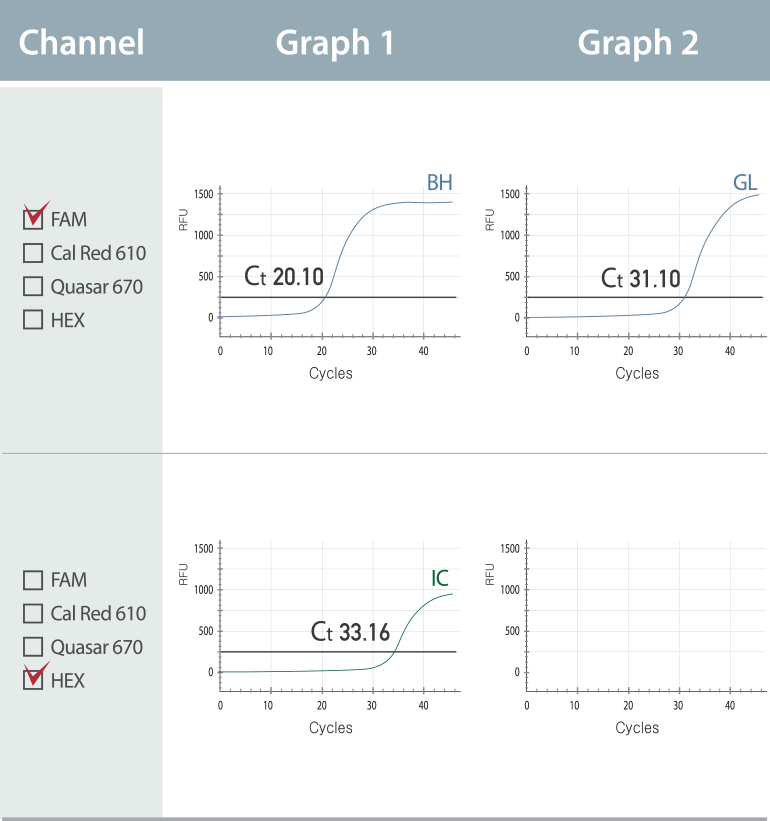
The result represents the co-infection of B. hominis(BH) G. lamblia(GL) in FAM channel with Ct values of 20.10 (Graph 1) and 31.10 (Graph 2), respectively.
