Allplex™ 2019-nCoV Assay is a multiplex Real-time PCR assay for simultaneous detection of 3 target genes of SARS-CoV-2 in a single tube. The assay is designed to detect RdRP and N genes specific for SARS-CoV-2, and E gene for all of Sarbecovirus including SARS-CoV-2.
Key Features and Benefits
-

Multiplex real-time PCR
Detection and identification of target genes (E gene, RdRP gene, N gene) specific for COVID-19 in a single tube
-
Short TAT
Results within 1 hour and 50 minutes after extraction
-
User-friendly workflow
Convenient workflow using Seegene’s automated one platform
-

Proper patient care
Accurate test results allow to quick and proper treatment
-
Powerful performance
PCR with high sensitivity and specificity
-

Automatic data analyzer
Automated data interpretation and LIS interlocking with Seegene Viewer
-

UDG system
Utilization of the UDG system to prevent carry-over contamination
-

Whole process validation
Whole process validation from extraction to PCR by whole process control
-
Analytes
Allplex™
2019-nCoV Assay (USA Only)
- Sarbecovirus (E gene)
- SARS-CoV-2 (N gene)
- SARS-CoV-2 (RdRP gene)
- Internal Control (IC)
-
Specimens
- Sputum
- Nasopharyngeal swab
- Oropharyngeal swab
- Anterior nasal swab
- Mid-turbinate
-
Ordering Information
ProductCat No. / SizeAllplex™ 2019-nCoV Assay (USA Only)RP10251Y / 50 rxns
RP10250X / 100 rxns
RP10252W / 124 rxns
Result
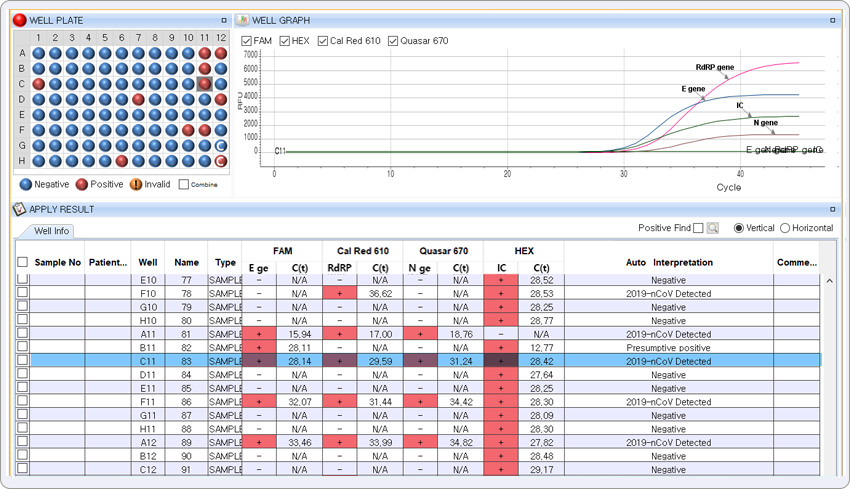
The highlighted result represents positive results for E gene of Sarbecovirus in FAM channel, RdRP gene and N gene of COVID-19 in Cal Red 610 and Quasar 670 respectively.
Note
Allplex™ 2019-nCoV Assay has not been FDA cleared or approved. This test has been authorized by FDA under an emergency use authorization for use by authorized laboratories. This test has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens. This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb- 3(b)(1), unless the authorization is terminated or revoked sooner.
