상단으로
Choose your location
Select your country or region to view content specific to your country.
Choose your country
This website contains information on products which is targeted to a wide range of audiences and could contain product details or information otherwise not accessible or valid in each country.
Positive reviews of Seegene’s HPV products in the global market
Oct 21, 2015

?
The 101st conference of the Korean Society of Obstetrics and Gynecology (KSOG) was held at the Grand Hilton Seoul from September 18 to 19. Attended by professors of gynecology from all over the country, the conference is a venue for sharing the latest medical trends and the results of a recent of studies.
This year’s KSOG conference included a presentation by a delegation from the National Cancer Center featuring a performance evaluation and comparison of FDA-certified products and Seegene’s HPV diagnostic products. According to the presentation, Seegene product is not only have the same quality degree of functionality as the FDA-approved products, but is the only product that provides additional test result information with the same price. Having verified the outstanding performance of Seegene products, the study and its results will be published as an article in the Annals of Clinical Biochemistry.
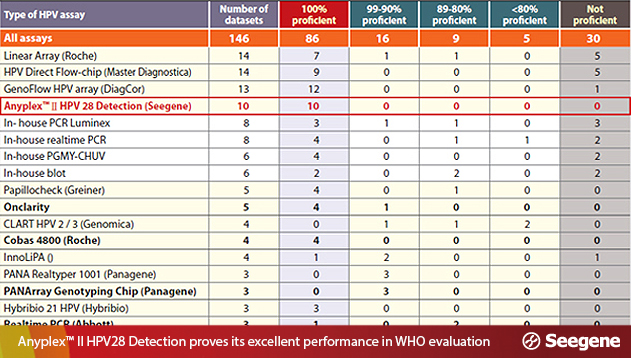
Presentations on the excellence of Seegene’s HPV products are also increasingly being made throughout the global presenters. In April, the research team of Dr. Chris JLM Meijer, the renowned Dutch pathologist, gave a presentation at the 2015 meeting of the European Society of Clinical Microbiology and Infectious Diseases on the results of a study that shows Seegene’s HPV products can be used to conduct screening tests for cervical cancer on not only current patients but also as a routine test for people. At the HPV 2015 conference, which was held in Portugal, there was even a presentation that asserted that Seegene’s HPV detection products achieved better results than similar products from world-class companies. The study results presented at this conference were given as a paper presentation by the WHO HPV Laboratory Network.
?
With the world’s most eminent scholars unanimously acknowledging the global influence of Seegene, a Korean company that is growing and evolving every day, the company is expected to build on its current reputation to become a world-class global diagnostics company.
