Choose your location
Select your country or region to view content specific to your country.
Allplex™ 2019-nCoV Assay is multiplex Real-time PCR assay for simultaneous detection of 3 target genes of SARS-CoV-2 in a single tube. The assay is designed to detect RdRP and N genes specific for SARS-CoV-2, and E gene for all of Sarbecovirus including SARS-CoV-2.
Key Features and Benefits
-
Short TAT
Results within 1 hour and 50 minutes after extraction
-

Multiplex real-time PCR
Detection and identification of target genes (E gene, RdRP gene, N gene) specific for COVID-19 in a single tube
-
User-friendly workflow
Convenient workflow using Seegene’s automated one platform
-
Powerful performance
PCR with high sensitivity and specificity
-

Proper patient care
Accurate test results allow to quick and proper treatment
-

Automatic data analyzer
Automated data interpretation and LIS interlocking with Seegene Viewer
-

Whole process validation
Whole process validation from extraction to PCR by whole process control
-
Analytes
Allplex™
2019-nCoV Assay
- Sarbecovirus (E gene)
- SARS-CoV-2 (N gene)
- SARS-CoV-2 (RdRP gene)
- Internal Control (IC)
-
Specimens
- Sputum
- Nasopharyngeal swab
- Nasopharyngeal aspirate
- Bronchoalveolar lavage
- Throat swab
-
Ordering Information
Result
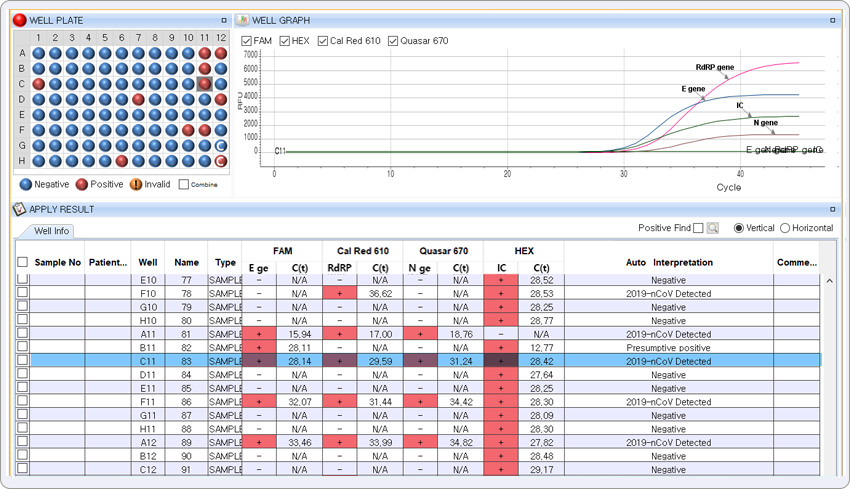
The result represents the positive result for E gene of Sarbecovirus in FAM channel, RdRP gene and N gene of COVID-19 in Cal Red 610 and Quasar 670, respectively.
Publication
Note
[1] For use with Seegene NIMBUS & STARlet only
