Choose your location
Select your country or region to view content specific to your country.
2020~2014
Our History
-

Dec,2020
Launched post-analysis platform "SG STATS"
-
Dec, 2020
Exceeded 1 trillion won in 2020 annual sales
-
Dec, 2020
Selected as 'Innovative Pharmaceutical Enterprise' (Ministry of Health and Welfare)
-
Jun, 2020
Expanded production facilities to "Songpa hangil building", "Hanam production center 1·2·3"
-

Jun, 2019
Establish a subsidiary - Seegene Brazil Diagnosticos LTDA - in Brazil
-
May, 2019
Obtained MDSAP certification
-
Oct, 2018
First successful development of molecular diagnosis reagents using SGDDS
-
Apr, 2018
Introduction of automated system for reagent tube capping and labeling
-
Jan, 2018
MOU with Tianlong, a Chinese Molecular Diagnosis Company for co-developing
-

Dec, 2017
Expand logistics facilities for MDx products
-

Nov, 2017
Establish a subsidiary - Seegene Germany GmbH - in Germany
-
Sep, 2017
MOU with Hamilton for co-developing
-
Jun, 2017
MOU with Mexico Government Agriculture Agency for Collaborative Development of Non-human MDx Assay
-
May, 2017
Collaboration with Thermo Fisher for FDA clearance of the MDx assays and instruments
-
Apr, 2017
Patent Seegene’s new generation of real-time technology (TOCE™) in the leading countries including the United States
-
Feb, 2017
Develop New Normalization Software Technology for molecular diagnosis
-
Jan, 2017
Present ‘All MDx Assays in One Platform’ solution at the 35th Annual J.P. Morgan Healthcare Conference
-
Dec, 2016
Head of the Seegene Middle East, awarded the Minister’s Award of Trade, Industry and Energy of Korea
-
Nov, 2016
Signed collaboration agreement with Hologic for developing high multiplex molecular diagnostic assays
Signed MOU with Institut Català d'Oncologia(ICO) in Spain for mutual cooperation -
Oct, 2016
Expansion of production facilities
-
Sep, 2016
Acquired Neoprobe for better custom oligo synthesis service
Launched SG Oligo designing service
President of Seegene Middle East, awarded a commendation from minister of Ministry of trade, industry and energy for ‘Merit in Exportation’ -
Jul, 2016
Signed MOU with DGIST for mutual cooperation
-
Jun, 2016
Launched SG Oligo designing system
Awarded In-Vitro Diagnostics Company of the Year at the 2016 Frost & Sullivan South Korea Excellence Awards -
May, 2016
Launched RUO products (Novaplex™) for U.S. market
Developed STARMag 96 X 4 Universal Cartridge Kit for nucleic acid extraction of multiple specimen types -

Apr, 2016
Establishing a joint venture with Biodist Group in Mexico: Biodist-Seegene Diagnostics
-
Sep, 2015
Signed the ODM supply agreement with BD(Becton, Dickinson)
Awarded the 2015 South Korea In-Vitro Diagnostics Company of the Year -

Jul, 2015
Established CANADA subsidiary
Signed CDx collaboration agreement with QIAGEN -

Jun, 2015
Established USA subsidiary
-
Feb, 2015
Receives FDA Clearance for its HSV Molecular Test
-
Dec, 2014
Selected as the ‘World class product of KOREA’ (Respiratory virus detection kit, STI pathogen detection kit)
-
Nov, 2014
Signed the first global ODM supply agreement with Beckman Coulter Diagnostics (Danaher)
-

Oct, 2014
Established subsidiaries - Seegene Middle East, in Dubai
-
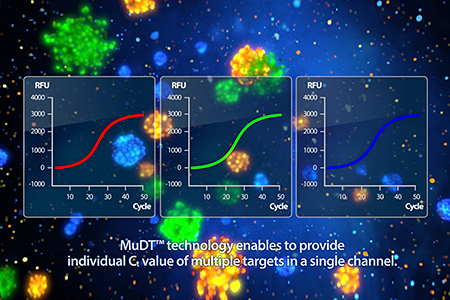
Jul, 2014
Developed MuDT™ (Simultaneous quantification and detection of multipletargets in a single fluorescence channel)
Licensed TOCE™ to Eisai(Japan) for companion diagnostics(CDx) -
Apr, 2014
Listed on KOSDAQ’s Hidden Champion (Korea Stock Exchange)
-

Jan, 2014
Established subsidiaries in Italy