Choose your location
Select your country or region to view content specific to your country.
Anyplex™Ⅱ HPV HR Detection simultaneously detects, differentiates and quantifies 14 high-risk HPV genotypes including HPV16 and HPV18 which are implicated as major risk factors for cervical cancer. Based on Seegene’s proprietary DPO™ and TOCE™ technologies, this assay performs on multiplex real-time PCR instrument and enables accurate screening test of HPV infection in a single reaction.
Key Features and Benefits
-

Multiplex real-time PCR
Detection of 14 high-risk HPV genotypes in a single reaction
-

Automatic data analyzer
Automated data interpretation and LIS interlocking with Seegene Viewer
-
User-friendly workflow
Convenient workflow using Seegene’s automated one platform
-

Quantitative analysis
Quantitative analysis by cyclic-CMTA
-
Useful monitoring
Useful tool for surveillance and infection control
-
UDG system
Utilization of the UDG system to prevent carry-over contamination
-
Proper patient care
Quick and proper treatment provided by accurate test results
-

Automatic data analyzer
Automated data interpretation and LIS interlocking with Seegene Viewer
-
Whole process validation
Whole process validation from extraction to PCR by whole process control
-
Analytes
Anyplex™ II
HPV HR Detection
14 High-risk HPV types- Human Papillomavirus 16 (HPV 16)
- Human Papillomavirus 18 (HPV 18)
- Human Papillomavirus 31 (HPV 31)
- Human Papillomavirus 33 (HPV 33)
- Human Papillomavirus 35 (HPV 35)
- Human Papillomavirus 39 (HPV 39)
- Human Papillomavirus 45 (HPV 45)
- Human Papillomavirus 51 (HPV 51)
- Human Papillomavirus 52 (HPV 52)
- Human Papillomavirus 56 (HPV 56)
- Human Papillomavirus 58 (HPV 58)
- Human Papillomavirus 59 (HPV 59)
- Human Papillomavirus 66 (HPV 66)
- Human Papillomavirus 68 (HPV 68)
- Internal Control (IC)
-
Specimens
- Liquid based cytology (e.g., ThinPrep® and Surepath™)
- Cervical swab
- Self-collected vaginal
-
Ordering Information
Result
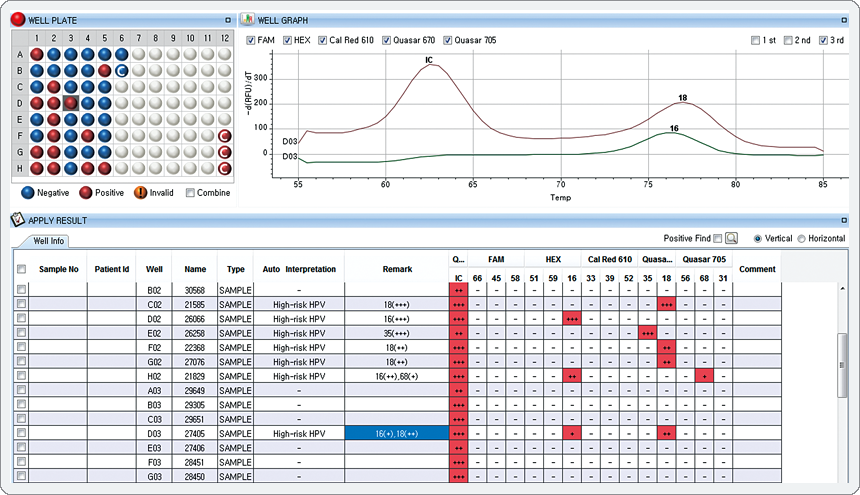
The result represents co-infection that HPV 16 is low (+) in the HEX channel, HPV 18 is intermediate (++) in the Quasar 670 channel.
