Choose your location
Select your country or region to view content specific to your country.
Choose your country
This website contains information on products which is targeted to a wide range of audiences and could contain product details or information otherwise not accessible or valid in each country.
High specificity & sensitivity
Seegene's products using proprietary technologies provide exceptional performance
Clinically validated by national authorities
Assessed from the 2013 and 2014 HPV LabNet international proficiency studies :
Continuing global improvement in human papillomavirus DNA genotyping services
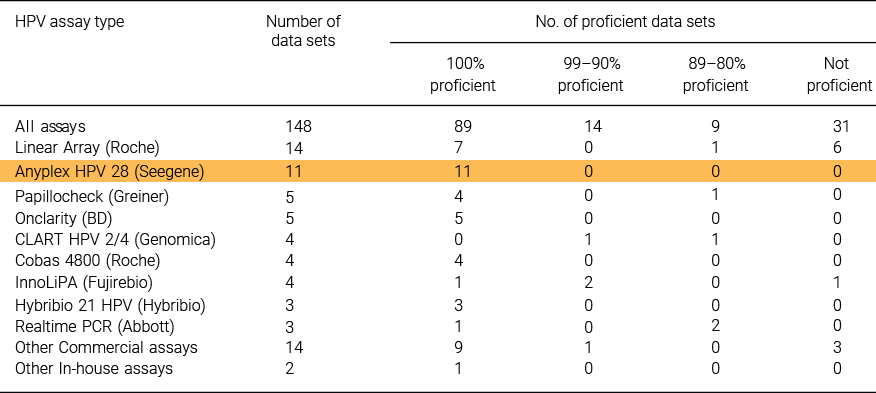
In 2014, 86/145 datasets (59%) that typed for at least one HPV type were 100% proficient for the types detected by the test. Of these, 32 datasets identified the content of all samples, including those with copy number amounts that were lower than required for proficiency.
Reference : Eklund C. et al. (2018) Journal of Clinical Virology, 101:74-84.Reference : Eklund C. et al. (2018) Journal of Clinical Virology, 101:74-84.
Results proved its reliability
Comparison of the fast track diagnostics respiratory 21 and Seegene Allplex™ RP assays 26 multiplex polymerase chain reaction assays for the detection of respiratory viruses
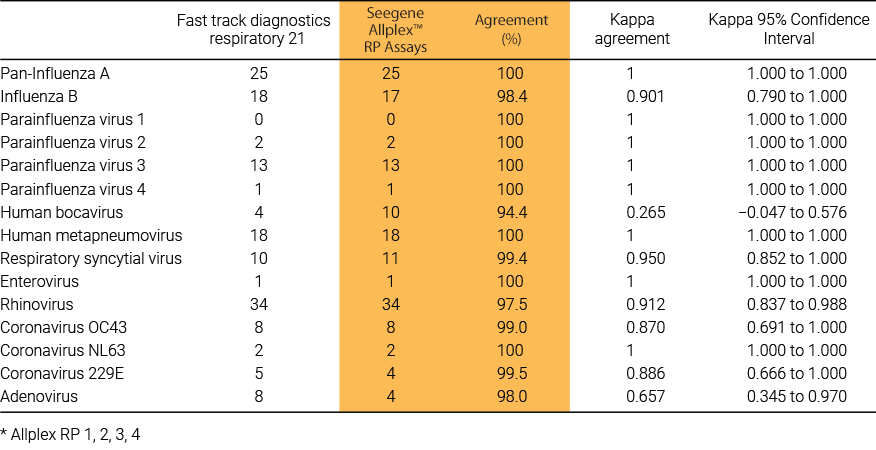
Although the performance of the assays was similar, the Seegene assay had the advantage of simultaneous detection of two gene targets for each of the common Influenza A subtypes, improved throughput of 30 samples per run and automated result analysis. Seegene assay had improved multiplexing ability compared to other real-time PCR methods with almost double the throughput compared to the FTD assay.
Reference : Barratt K. et al. (2017) British Journal of Biomedical Science.
Studies showed its exceptional performance
Comparison studies of bacterial gastrointestinal infection with routine diagnostic methods.
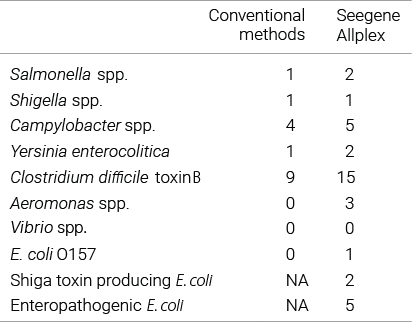
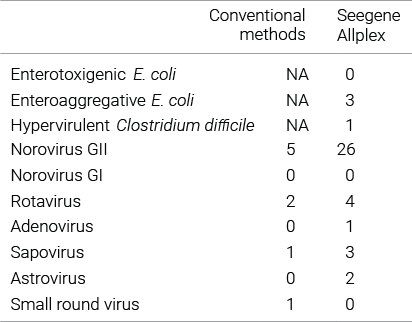
For bacterial pathogens, Allplex™ was able to detect the organisms grown in culture with high sensitivity and additionally detected several types that could not be detected with conventional culture methods. Overall, Allplex™ had a > 2-fold higher detection rate than the conventional methods.
Reference : Amrud K. et al. (2018) BMC Research Notes, 11:514.
