Choose your location
Select your country or region to view content specific to your country.
More advanced solution for COVID-19 and variants
Seegene is playing a critical role in developing effective testing solution to mitigate the spread of rapidly mutating COVID-19 and variants.
Full Screening and Identification
for SARS-CoV-2 and Variants

Full screening for SARS-CoV-2 and Variants
Full screening for SARS-CoV-2 and variants
including the five notable mutations in S gene
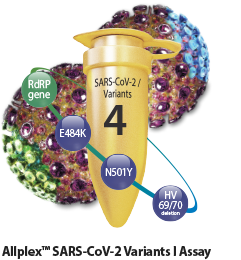
Identification of SARS-CoV-2 Variants
Identification of major SARS-CoV-2 variants of
concern and highly conserved region in RdRP gene
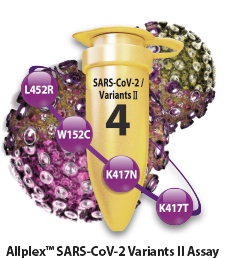
Identification of SARS-CoV-2 Variants
Identification of major SARS-CoV-2
variants of concern
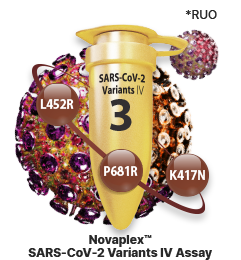
Identification of SARS-CoV-2 Variants
Accurate detection of SARS-CoV-2
Delta variants
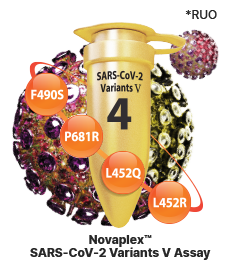
Identification of SARS-CoV-2 Variants
Accurate detection of SARS-CoV-2
Delta & Lambda variants

Identification of SARS-CoV-2 Variants
Accurate detection of SARS-CoV-2
Lamda & Mu variants
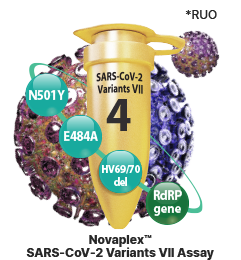
Identification of SARS-CoV-2 Variants
Accurate detection of SARS-CoV-2
and Omicron-specific mutations
Move Forward to Defeat SARS-CoV-2 Variants

Seegene utilizes bioinformatics (SG-Insilico™) to monitor newly emerging SARS-CoV-2 sequence information on a regular basis and design optimized diagnostic assay.
Multiplex Real-time PCR assay portfolio
for COVID-19 and Variants
Analytes
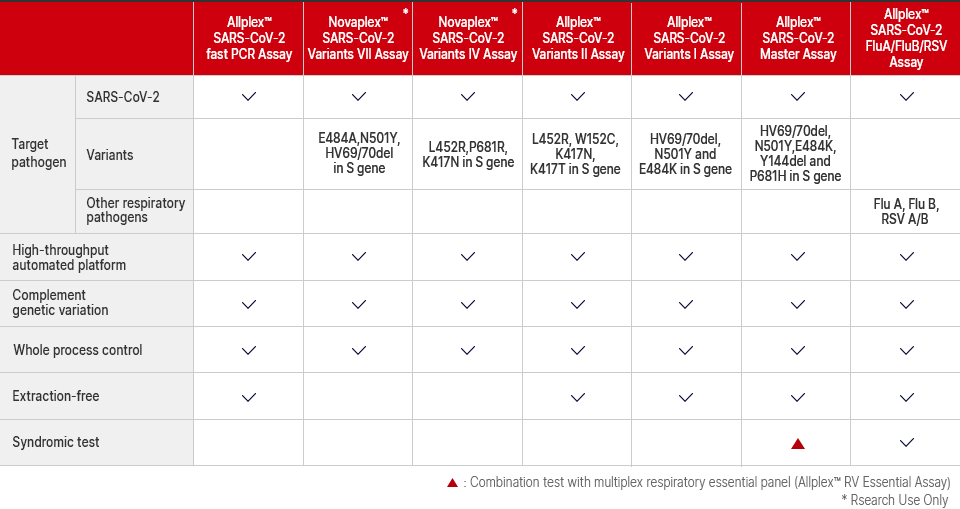
Allplex™ 2019-nCoV Assay
- - N gene
- - RdRP gene
- - E gene
- - Exogenous internal control (IC)
Allplex™ SARS-CoV-2 Assay
- - N gene
- - RdRP/S gene
- - E gene
- - Exogenous internal control (IC)
Allplex™ SARS-CoV-2/FluA/FluB/RSV Assay
- - N gene
- - RdRP/S gene
- - Influenza A
- - Influenza B
- - RSV A/B
- - Endogenous internal control (IC)
- - Exogenous internal control (IC)
Allplex™ SARS-CoV-2 Master Assay
- - E gene
- - N gene
- - RdRP/S gene
- - HV 69/70 deletion, N501Y, E484K, Y144 deletion and P681H in S gene
- - Endogenous internal control (IC)
Allplex™ SARS-CoV-2 Variants I Assay
- - RdRP gene
- - E484K in S gene
- - N501Y in S gene
- - HV69/70 deletion in S gene
- - Endogenous internal control (IC)
Allplex™ SARS-CoV-2 Variants II Assay
- - L452R in S gene
- - W152C in S gene
- - K417N in S gene
- - K417T in S gene
- - Endogenous internal control (IC)
Novaplex™ SARS-CoV-2 Variants IV Assay (RUO)
- - L452R in S gene
- - P681R in S gene
- - K417N in S gene
- - Endogenous internal control (IC)
Novaplex™ SARS-CoV-2 Variants V Assay (RUO)
- - F490S in S gene
- - P681R in S gene
- - L452Q in S gene
- - L452R in S gene
- - Endogenous internal control (IC)
Novaplex™ SARS-CoV-2 Variants VI Assay (RUO)
- - L452Q in S gene
- - F490S in S gene
- - R346K in S gene
- - D950N in S gene
- - Endogenous internal control (IC)
Novaplex™ SARS-CoV-2 Variants VII Assay (RUO)
- - E484A in S gene
- - HV69/70 deletion in S gene
- - N501Y in S gene
- - RdRP gene
- - Endogenous internal control (IC)
Allplex™ SARS-CoV-2 fast PCR Assay
- - E gene
- - N gene
- - RdRP gene
- - Endogenous internal control (IC)
Summary of Seegene's COVID-19 assay
Expanded selectivity of syndromic test
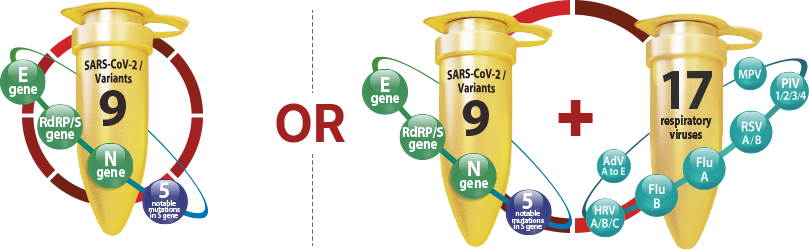
Allplex™ SARS-CoV-2 Master Assay can be used alone or expanded to syndromic test with respiratory essential panel for common respiratory infections, including influenza A and B, metapneumovirus, parainfluenza virus 1/2/3/4, adenovirus, rhinovirus A/B/C and respiratory syncytial virus A/B, to provide more insights for diagnosis of symptomatic patients especially with the emerging SARS-CoV-2 variants.
Primary screening for COVID-19, Flu and RSV
Allplex™ SARS-CoV-2/FluA/FluB/RSV Assay is designed to detect and differentiate between SARS-CoV-2, influenza A, influenza B and RSV
for primary screening during the upcoming flu season.

More reliable results using dual internal controls

- The endogenous and exogenous internal controls are designed to verify the entire process from sample collection to nucleic acid extraction and PCR steps
- The endogenous internal control enables validating correct sampling for self-collected specimens
Increased accuracy by minimizing risks
Multiplex real-time PCR with high sensitivity and specificity by utilization of Seegene’s proprietary technologies
Detecting N, S, RdRP and/or E target genes of SARS-CoV-2 to minimize the risk of genetic variations and allow high accuracy

No cross-reactivity confirmed with respiratory pathogens including SARS, MERS and SARS-related viruses
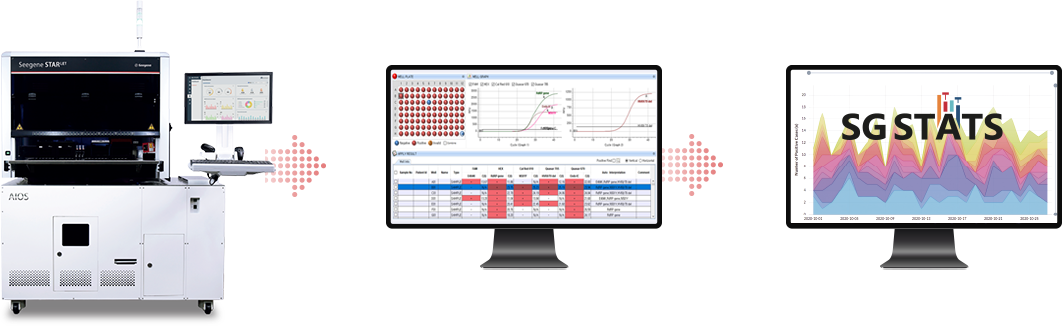
Full automation system
Seegene’s automated molecular diagnostic system allows a unique streamlined workflow with quick and simple steps for detection of COVID-19.
View video clips
Watch the videos that can show you the outstanding features and benefits of Seegene’s COVID-19 assays.
Kick your Real-time PCR up a notch!
With Seegene’s core technologies and proprietary know-hows, enhance your MDx products with incomparable performance and usability.

Oligo Design
Oligo design technology for high multiplex PCR: SG-Insilico™
Enables rapid designing of high multiplex oligos using Seegene-developed algorithms and confidential variables.

Amplification
Highly specific amplification technology for high multiplex PCR: DPO™
Allows the implementation of high multiplex diagnostics with unparalleled specificity using Seegene’s unique primer structure.
Target Detection
High multiplex target detection technology: TOCE™
Achieves accurate target signals by independently controlling multiple target signals for primer and probe annealing temperatures.
Quantitative
Detection
High multiplex target detection & quantification technology: MuDT™
Broadens capacity for high multiplex target detection & quantification by enabling analysis of multiple Ct values in a single detection channel.

Interpretation
High multiplex signal processing technology: DSP™
Enables accurate reporting of target’s positive or negative results through Seegene’s proprietary signal processing algorithms.
Note
[1] Allplex™ 2019-nCoV Assay has not been FDA cleared or approved. This test has been authorized by FDA under an emergency use authorization for use by authorized laboratories. This test has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens. This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb- 3(b)(1), unless the authorization is terminated or revoked sooner.
[2] Allplex™ SARS-CoV-2/Flu A/Flu B/RSV Assay, SARS-CoV-2 Assay and RV Essential Assay are intended for in vitro diagnostic use in Europe and not available in all countries.
